Based on recent experimental findings. How is the amino acid held on.

Learn Translation And Translation Machinery In 2 Minutes
Establishing the correct reading frame by the ribosome.
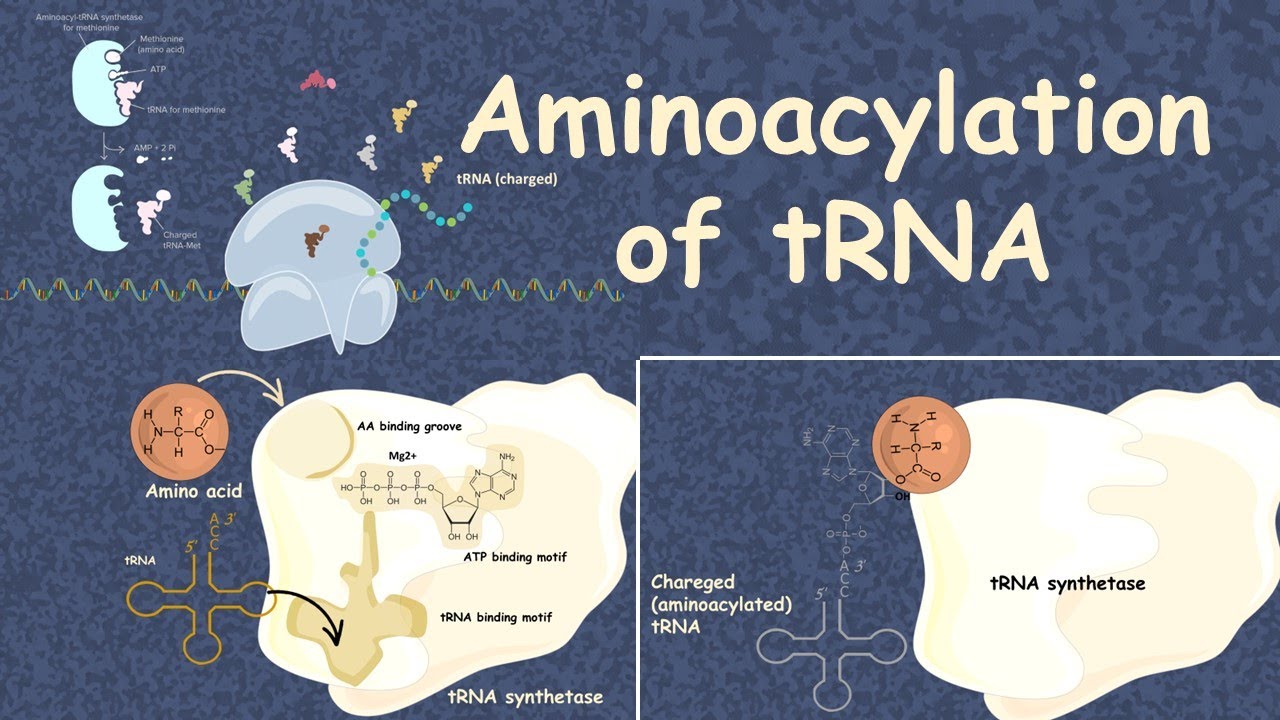
. The reaction proceeds in two steps. Aminoacyl Trna Synthetase Hd Animation On Vimeo Aminoacyl-tRNA synthetases ARSs are essential and ubiquitous house-keeping enzymes responsible for charging amino acids to their cognate tRNAs and providing the substrates for global protein synthesis. Aminoacyl-tRNA synthetases are essential enzymes required for translation.
Aminoacyl-tRNA synthetases ARSs are generally considered as housekeepers involved in protein synthesis whose primary function is to catalyze the aminoacylation of transfer RNAs tRNAs. 2 Chemical modifications of substrates can have comparable influence on catalysis as can changes in assay conditions. Mutations in the mitochondrial-specific arginyl-tRNA synthetase RARS2 have been shown to induce progressive pontocerebellar atrophyhypoplasia.
3 All enzymes show a specificity for the 2- or 3-position of the tRNA. Binding of charged tRNA to the ribosome A site b. Although aminoacyl-tRNA synthetases ARSs are housekeeping enzymes essential for protein synthesis they can play non-catalytic roles in diverse biological processes.
Their accretive addition to virtually all aaRSs correlates with the progressive evolution and complexity of eukaryotes. Each enzyme is exquisitely adapted to covalently link a single standard amino acid to its cognate set of tRNA isoacceptors. Follow NCBI Connect with NLM.
The aminoacyl adenylate remains associated with the synthetase enzyme where in the second step it. Recent studies have revealed a role of multiple ARSs in pathology and their potential use as pharmacological targets and therapeutic reagents. The exact etiology for its organ specificity remains unclear.
Aminoacyl tRNA synthetase 1. Catalysis of a new peptide bond from the growing polypeptide to the next amino acid c. A common picture emerges.
TRNA synthetases catalyze the ligation of tRNAs to their cognate amino acids in an ATP-dependent manner. In the first step A the amino acid blue is activated with ATP red in the synthetase active site not depicted forming the aminoacyl-AMP and releasing PP iB The amino acid is transferred to the tRNA green and AMP is released depicted in the image transfer to the 3-OH characteristic of class I aaRS while in class II transfer happens at the 2. COG Funtional Cat COG symbol Name EC.
Similarity networks for the whole set of Aminoacyl-tRNA synthetases reflect the tree topology of each. Some ARSs are capable of forming complexes with each other and additional proteins. In humans the 20 different types of aa-tRNA are made by the 20 differe.
Aminoacyl-tRNA synthetases play a key role in protein biosynthesis by catalyzing the specific aminoacylation of tRNA at their 3 ends a two step reaction termed charging of tRNA. In this video we have discussed the tRNA Charging or Aminoacylation in ProkaryotesThis reaction is catalyzed by aminoacyl tRNA synthetasesAn aminoacyl-tRN. Aminoacyl tRNA Synthetase Somanna A.
To address the global threat in-depth investigations in pathogenesis are essential for interventions in. First amino acid and ATP form an aminoacyl adenylate molecule releasing pyrophosphate. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
An aminoacyl-tRNA synthetase aaRS or ARS also called tRNA-ligase is an enzyme that attaches the appropriate amino acid onto its corresponding tRNA. It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. The aminoacyl-tRNA synthetases are prominently known for their classic function in the first step of protein synthesis where they bear the responsibility of setting the genetic code.
Which of the following is the actual event that translates the language of nucleic acids the sequence of bases A T U C and G to the language of proteins determining which amino acid will be added to the polypeptide. The aminoacyl-tRNA synthetases are prominently known for their classic function in the first step of protein synthesis where they bear the responsibility of setting the genetic code. Aminoacyl-tRNA synthetases ARSs are essential and ubiquitous house-keeping enzymes responsible for charging amino acids to their cognate tRNAs and providing the substrates for global protein synthesis.
1 The enzymes work with different catalytic cycles kinetic constants and specificities under different assay conditions. These ancient enzymes have evolved idiosyncratically to host alternate activities. Attachment of the appropriate amino acid to the tRNA by aminoacyl tRNA synthetase.
2 steps of aminoacyl tRNA charging. Infectious diseases such as the ongoing coronavirus disease 2019 COVID-19 continue to have a huge impact on global health and the host-virus interaction remains incompletely understood. Release of the finished polypeptide at the stop codon d.
This enzymatic reaction is conserved and proceeds mainly in two steps 1 2. Classes of Aminoacyl tRNA Synthetases 5. National Library of Medicine 8600 Rockville Pike Bethesda MD 20894.
First an amino acid and an adenosine triphosphate ATP molecule bind to. Aminoacyl-tRNA synthetases ARSs are a family of 20 essential enzymes one for each amino acid that ligate amino acids to their corresponding tRNAs in protein synthesis Fig. Over the course of evolution eukaryotic aminoacyl-tRNA synthetases aaRSs progressively incorporated domains and motifs that have no essential connection to aminoacylation reactions.
This is Aminoacyl tRNA synthetase HD Animation by PITB on Vimeo the home for high quality videos and the people who love them. This characteristic is most pronounced in mammals which produce a macromolecular complex comprising nine. Attachment of the appropriate amino acid to the tRNA by aminoacyl tRNA synthetase e.
A Adenylylation of amino acid b Transfer of adenylylated amino acid to tRNA 3. Class I tRNA synthetases attach an appropriate amino acid to the 2 oxygen of the 3 terminal residue and class II synthetases attach amino acids at the 3 oxygen. Lysyl-tRNA synthetase class I.
Each enzyme is exquisitely adapted to covalently link a single standard amino acid to its cognate set of tRNA isoacceptors.
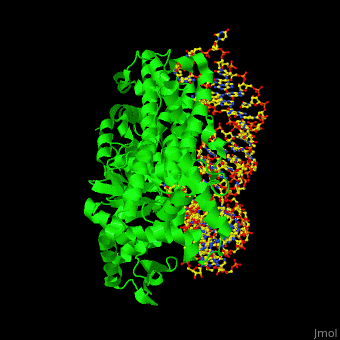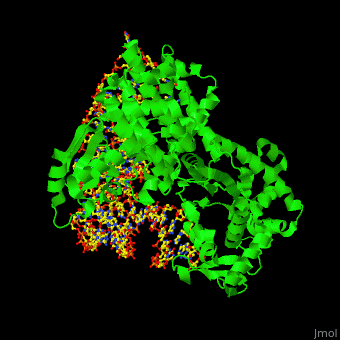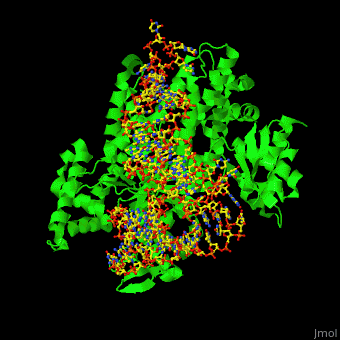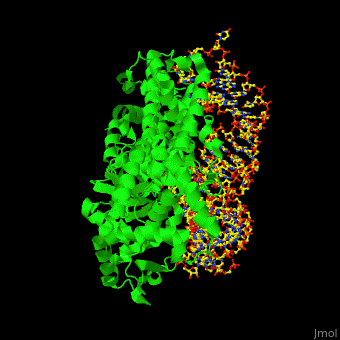
Aminoacyl Trna Synthetase Proteopedia Life In 3d

Translation Part 3 Of 8 Aminoacyl Trna Synthetase Reaction Youtube
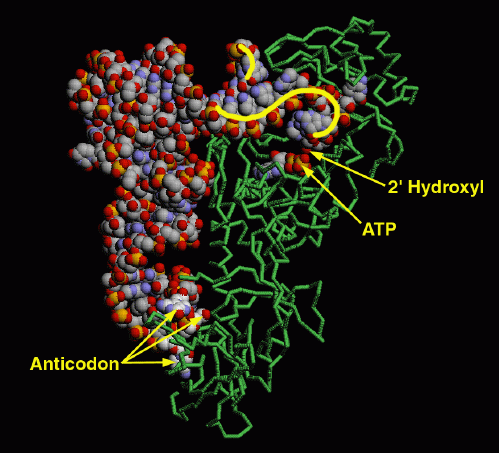
Virtuelle Nano Welten Pdb Molecule Of The Month 16 Aminoacyl Trna Synthetase

Aminoacylation Of Trna Translation 101 Youtube
Aminoacyl Trna Synthetase Hd Animation On Vimeo

Aminoacyl Trna Synthetase Part 1 Youtube
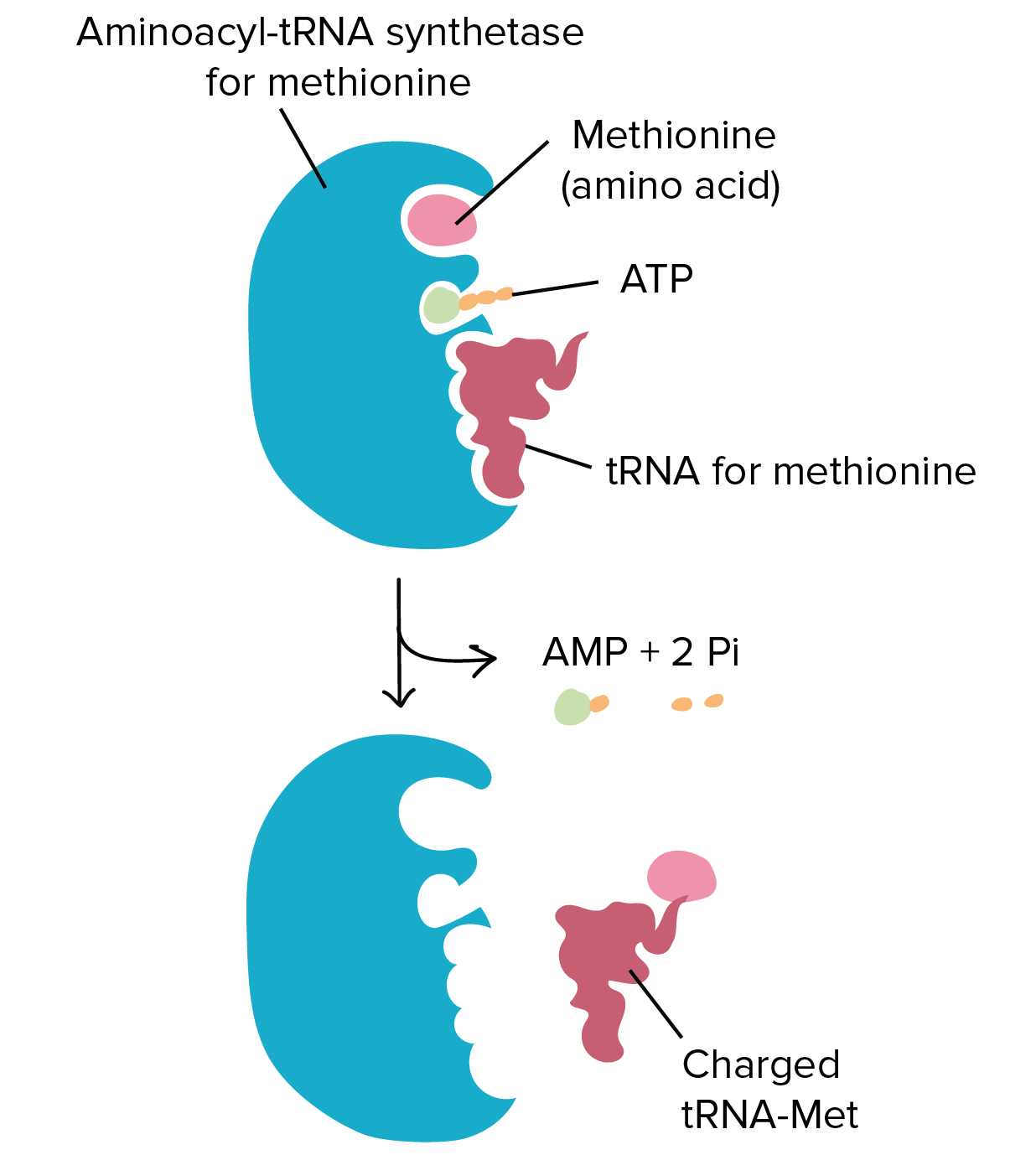

0 comments
Post a Comment